COVID-19 Risk Evaluation model – CORE
Host institution
Center for Interdisciplinary Innovation and Research – Aristotle University of Thessaloniki
HERACLES Research Center on the Exposome and Health
Principal Investigator
Prof. Dimosthenis A. Sarigiannis (e-mail: sarigiannis@auth.gr)
Background
Curbing the global spread of SARS-CoV-2 requires implementation of multiple population-wide strategies; in this context it is essential to know how the timing and stringency of such measures will affect ‘flattening the curve’. We have proposed a new model that predicts the evolution of epidemics and helps to assess the impact of different strategies to contain the spread of the infection, including lockdown and social distancing, as well as testing and contact tracing.
Model link
https://envelab.shinyapps.io/covid19-seirx/
Model description
The HERACLES research group on the Exposome and Human Health of the Aristotle University of Thessaloniki Center for Interdisciplinary Research and Innovation (KEDEK), in collaboration with the University School of Advanced Studies IUSS in Pavia have developed a computational tool for the evaluation of the public health risk from the COVID-19 epidemic in Greece and Italy and evaluated the effectiveness of different non-pharmacological intervention scenarios for public health risk management.
The computational tool for public health risk management from COVID-19 is called CORE: COVID Risk Evaluation model. It includes an advanced model of the spread of the epidemic, which is an evolution of the most advanced SEIR models available [1], also taking into account the implementation dynamics of non-pharmacological interventions such as virus detection testing geared towards the general population or targeted sub-population groups, circulation restriction and population containment measures [2], in the evolution of the dispersion (described as compartment X) and the final health risk assessment of the affected population.
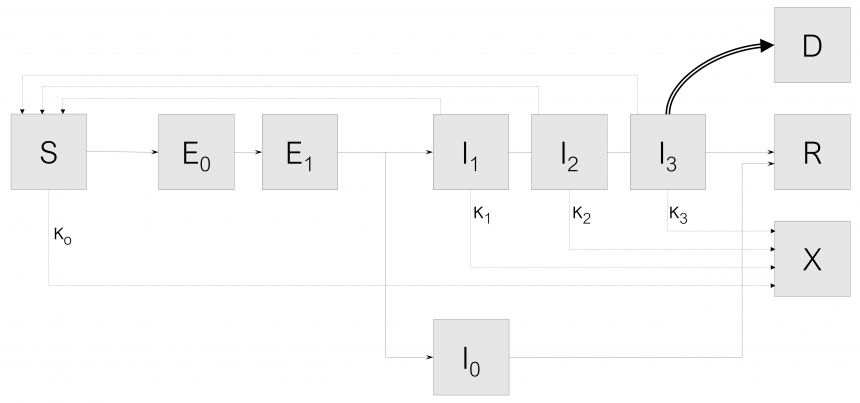
The SEIR-Xms dispersion model, which is also the dispersion calculation engine, has been extended to a multi-state population model to describe in detail the different possible states of the population based on the typology and severity of the symptoms of the disease (SEIR-X multi-state model, or SEIR-Xms). This computational tool has been successfully applied in all regions of Northern Italy and in Greece so far. The graphical representation of the SEIR-Xms model is shown in Figure 1.
Fig.1: System configuration of the CORE model for COVID-19 risk management
The system configuration capturing the interactions among these states of the population are shown in Fig. 1. We omit the probability rate of becoming susceptible again after having recovered from the infection. Although anecdotal cases are found in the literature, the reinfection rate value appears negligible. Mathematical models that describe the dynamics of infectious diseases at the population level are usually based on the classical framework of sensitive (S) – infected (I) – treated (R) – SIR [1]. In SIR models, individuals are divided into compartments based on their state of infection progression. Thus, each person may be in only one state at a given time, although they may go through different states during the evolution of the disease. SEIR models incorporate an additional state of the population, where infected individuals are exposed but not directly infectious (compartment E). The existence of this state of the infected population is a more biologically valid hypothesis for most diseases, as individuals typically face some level of delay between the time of infection and the time they become infectious. In addition, in this model an extension was made with an additional population state (X), which describes the individuals who have been in some form of restriction and do not participate in the spread of the disease. This approach is more accurate in capturing the real dynamics of the impact of population reduction measures on disease transmission due to reduced contact, rather than a virtual reduction in the rate of transmission. The variability of SARS-CoV-2 transmissivity is subject to environmental factors such as temperature [3], humidity [4] and ultraviolet radiation [5], incorporating thus explicitly the influence of seasonal variation on the spread rate of the infection and allowing the application of the CORE tool to settings at different latitudes and climatic characteristics. Moreover, the effect of viral load related to the various infection stages on transmissibility is accounted for as well [6].
In addition, in this model, the dynamics of infection of susceptible individuals (S), describes the possibility that exposed individuals who have not yet developed symptoms may be able to transmit the virus (“pre-symptomatic transmission”), therefore, the category of exposed (E) is divided into two separate classes, E0 (without symptoms or transmission) and E1 (without symptoms but can transmit). Infected people (I) are described in three different categories: (a) infected people start with a mild infection (I1), from which they either recover and enter the R compartment, or progress (b) to a serious infection (I2). People with a serious infection either recover and go to compartment R, or get worse and go into critical condition in ICU (I3). People in critical condition either improve and return to category I2 until they recover or die (compartment D). In addition, as far as the transition from exposure to infection is concerned, the possibility of asymptomatic infection is also included. After exiting category E1, a fraction f of the exposed population develops asymptomatic infection (compartment I0), while the remaining fraction 1-f develops a symptomatic infection (inserted in compartment I1). Asymptomatic infection (IO) never progresses to more severe stages and progresses to recovery (R).
Data from various recently published studies have been used to configure the model in which the specific characteristics of the virus have been studied which determine its dispersion, such as contagion b, incubation time, time for which one remains in the vulnerable population group, and time (c-1) for which one remains ill until one goes to the group of those who return and acquire immunity (or emigrate from the community).
The impact of non-pharmacological interventions is captured by a series of coefficients describing the reduction in probability of contacts associated with the containment or quarantine measures analyzed (κ0); these coefficients are applicableon both the I and S population segment. These rates are time-varying, so as to reflect the impact of the restrictive measures imposed on the respective implementation dates of the measures. The effectiveness of the measures to restrict the circulation and isolation of patients was determined by adapting the development curve of the dispersion to the data of confirmed cases, as recorded by the EODY 5. Based on the above, the course of SARS-CoV-2 dispersion in Greece was calculated by the SEIR-Xms model. In order to analyze the data correctly and estimate the actual level of expansion of the epidemic in the whole population of the country, the number of confirmed cases was multiplied by a factor, which is based on the results of an international study taking into account the mortality of the virus (approximately 1.4%) and the time lag between transmission and death (approximately 13) days) 1, for Greece it has been determined to range between 8.5 (for the first days of confirmed cases) to 4.2 (for the last days where the dispersal has been limited and more checks have been carried out). For comparison, the corresponding rate in Italy in the first days of the phenomenon was equal to 16.
Main findings and limitations
According to the CORE model estimates, measures currently in place prove to be particularly effective and have dramatically reduced the spread of the epidemic. With continuation of their implementation (and compliance by the population), the number of new cases is gradually decreasing from the first days of April, as well as the number of cases in critical condition that need hospitalization in ICU. The overall good course of the dispersal restriction in Greece leaves room for a gradual reduction of the restriction measures. However, lifting must be done progressively to avoid the risk of resurgence by launching a new cycle of contagion that will cancel the benefits of the hitherto successful effort. A short extension of the lockdown would lead to a faster lifting of the total restrictive measures. Testing is important because undetected infected people, most of whom are asymptomatic, largely sustain the epidemic spread.
The CORE computational engine is currently being updated through the design and implementation of a user-friendly GUI to enable policymakers use it readily for policy scenario development and exploration. This will allow them to have the possibility to evaluate quantitatively the effect of each individual measure (either for containment or for lifting the already enforced containment measures) on overall public health risk from SARS-CoV-2. Our findings aim to provide policymakers with a tool to assess the consequences of possible strategies, including lockdown and social distancing, as well as testing and contact tracing both during phase 1 (the epidemic suppression phase) and phases 2 and 3 (the transition towards normalcy). Our simulation results, achieved by combining the model with the available data about the COVID-19 epidemic in Greece and Italy, suggest that enforcing strong social distancing measures is urgent, necessary and effective. The earlier the lockdown is enforced, the stronger the effect obtained. CORE model results also underline the benefits of mass testing, whenever facilities are available.
References
[1] J.C. Blackwood and L.M. Childs, An introduction to compartmental modeling for the budding infectious disease modeler. Letters in Biomathematics, 2018. 5(1): p. 195-221.
[2] B.F. Maier and D. Brockmann, Effective containment explains sub-exponential growth in confirmed cases of recent COVID-19 outbreak in Mainland China. medRxiv, 2020: p. 2020.02.18.20024414.
[3] B. Oliveiros, L. Caramelo, N. Ferreira, and F. Caramelo, Role of temperature and humidity in the modulation of the doubling time of COVID-19 cases. 2020.
[4] J. Wang, K. Tang, K. Feng, and W. Lv, High Temperature and High Humidity Reduce the Transmission of COVID-19. 2020.
[5] W. Kowalski, T. Walsh, and V. Petraitis, 2020 COVID-19 Coronavirus Ultraviolet Susceptibility. Report number: COVID-19_UV_V20200312. DOI: 10.13140/RG.2.2.22803.22566. 2020.
[6] X. He, E.H.Y. Lau, P. Wu, X. Deng, J. Wang, X. Hao, Y.C. Lau, J.Y. Wong, Y. Guan, X. Tan, X. Mo, Y. Chen, B. Liao, W. Chen, F. Hu, Q. Zhang, M. Zhong, Y. Wu, L. Zhao, F. Zhang, B.J. Cowling, F. Li, and G.M. Leung, Temporal dynamics in viral shedding and transmissibility of COVID-19. Nature Medicine, 2020. 26(5): p. 672-675.