The INTERA project
To this aim ENVE-Lab developed a broadly applicable, comprehensive and easily accessible indoor exposure and risk assessment methodology based on a full mechanistic approach along the sources-to-dose continuum.
The methodology designed to assess aggregate exposures to chemicals in the indoor environment has been implemented in a flexible and user-friendly web-based modelling platform which is a key component of project ad that allows the inter-connection between the steps of the full-chain assessment.
The modelling platform is accessible via a web-based user interface http://www.intera.cperi.certh.gr/main.php and includes five main modules:
Indoor Air Quality module, linking sources to indoor concentrations, taking into account the physicochemical processes in indoor settings: dispersion, ventilation, gas-particle-dust partitioning, etc.
Exposure module including several models for the dermal, inhalation and oral routes, taking into account time-µenvironment-activity patterns and inhalation rates based on activity, gender and body weight.
Internal dosimetry module, which computes aggregate exposure linking temporal patterns to internal dose through a generic Physiology Based PharmacoKinetic (PBPK) model. It estimates the internal doses of contaminants and their metabolites at the target tissue lowing assimilation of biomarker data, which is emerging from national and European biomonitoring programs.
Uncertainty and variability of exposure and risk determinants are assessed along the full-chain assessment through hierarchical modelling using Markov Chain Monte Carlo technique.
Database module containing several types of data ranging from human physiological parameters to emission data. Data are stored along with their geographical information in order to allow users to build realistic exposure scenarios to represent typical exposure conditions for specific countries and/or cities in Europe.
Figure. INTERA software interface.
The INTERA methodology was applied and tested in three different case studies selected on the basis of relevance and novelty of the issue, different and multiple pathways and routes of entry and different chemical. These were DMF (dimethyl fumarate, dermal route), phthalates (multi-pathway exposures), and BTEX (benzene, toluene, ethylbenzene and xylenes, with mixture effect).
The overall scope was to derive population exposure levels to these chemicals (both external and internal) in indoor settings according to different geographical locations in Europe.
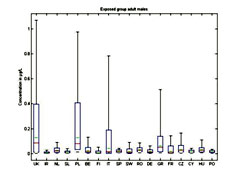
Figure 4. Whisker plot of benzene metabolite (BO, PH, HQ) concentration in bone marrow for adult males (max, min, 95%, 5%, median (red) and mean (green) estimates)
The project was completed in April 2012.
The TAGS Project
The methodology for quantitative aggregate exposure assessment is implemented into a computational platform, the core of which is a synthetic dynamic modeling environment able to track and describe in mathematical terms all the steps of the full chain approach, implementing both mechanistic (e.g. dispersion models, Physiology Based ToxicoKinetic Models) and probabilistic methodologies (Markov Chain Monte Carlo or maximum likelihood estimates) based on outcome optimization and the current status of knowledge and data availability. The project was completed in April 2012.
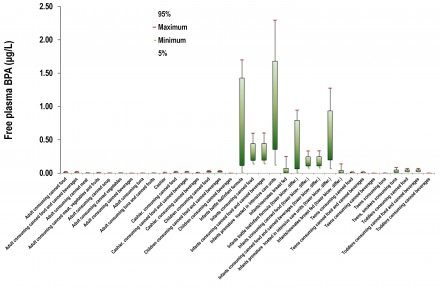
A very interesting application of the project was the bisphenol-A (BPA) case study. BPA, is an organic compound with two phenol functional groups. It is used to make polycarbonate plastic and epoxy resins, along with other applications. Since BPA is a known estrogenic, an increasing concern about the use of BPA in consumer products starts to rise since 2008. As a result, several governmental regulatory bodies issued questioning on its safety, while in 2010 the United States Food and Drug Administration (FDA) raised further concerns regarding exposure of fetuses, infants and young children. In the European Union, BPA use is banned in baby bottles since mid-2011. BPA is subjected to 2 major controversies that regard the actual toxicological threshold that needs to be taken into account as well as its BPA toxicokinetic behavior. Within the TAGS methodology, BPA was investigated as a case study.
Exposure assessment of several exposure scenarios including multiple pathways (releases in the environment during production and processing, transfer through food chain, uses in consumer products e.g. baby bottles and medical equipment), and routes (oral, inhalation and dermal) indicates that there is no scenario of concern
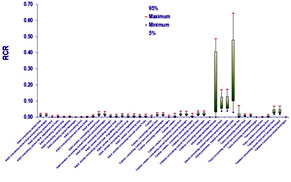
Figure. Risk Characterization Ratio under several environmental/consumer exposure scenarios.
However, incorporation of more advanced modeling tools and toxicokinetic considerations substantially facilitated by our generic two generation PBTK model, revealed significant bioavailability differences. These are attributed to a) the ontogeny of the enzymes employed to BPA detoxification and b) due to the lack of first pass metabolism when BPA is inhaled. Under this refined assessment two exposure scenarios were identified as potentially problematic, namelly infant milk formula bottle-fed neonates and neonates hosted in intensive care units